<CNS autoimmune disease therapy by CTLA-4 signaling peptide>
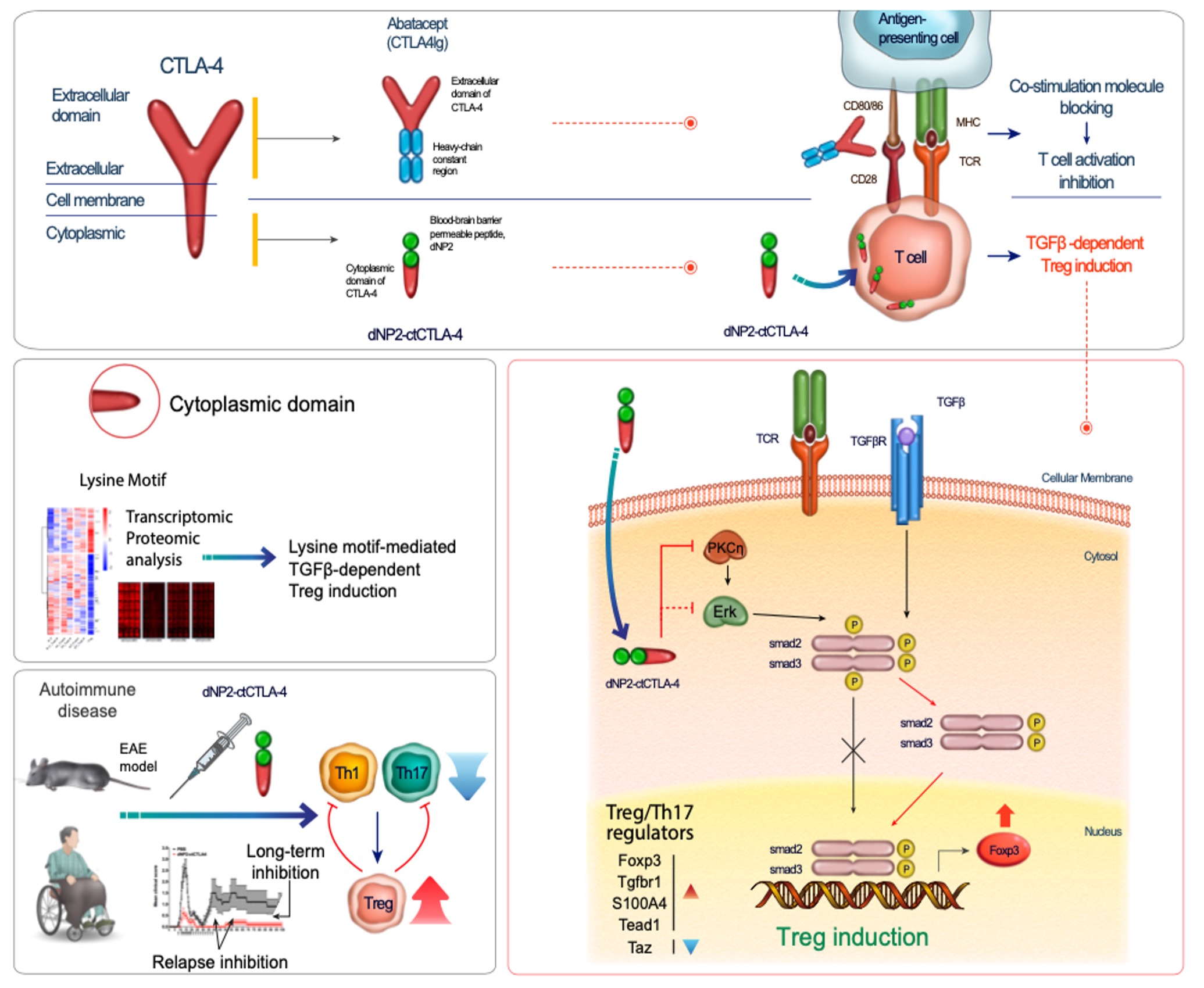
Regulatory T (Treg) cells play a key role in immune tolerance to self-antigen and prevents autoimmune disease. However, none of immune modulatory drugs have been approved for human trial yet based on in vivo Treg generation. CTLA-4 is an immune checkpoint molecule, which competes with CD28 for negative regulation of T cell activation and also it is a crucial molecule for regulatory T cell functions. Extracellular domain of CTLA-4 has been utilized for masking B7 molecules in antigen-presenting cells by conjugation to human immunoglobulin Fc (CTLA-4Ig) for treating autoimmune diseases like rheumatoid arthritis. However, the importance of its cytoplasmic signaling in T cells has not been much highlighted although there is a ligand independent functional ability of CTLA-4. We generated cytoplasmic domain of CTLA-4 (ctCTLA-4) protein conjugated with a Blood-brain barrier-penetrating peptide dNP2 that we previously discovered. This ligand independent CTLA-4 signaling protein (dNP2-ctCTLA-4) or synthetic peptide could inhibit infiltration of Th1 and/or Th17 cells with increased Treg cells in the spinal cord that alleviates autoimmune encephalitis. BBB penetration by dNP2 enhances ctCTLA-4 functions. We propose that it could enhance TGF-b signaling with increase of nuclear localization of smad2/3 via PKC-eta interaction to inhibit ERK phosphorylation. In addition, dNP2-ctCTLA-4 could inhibit human T cell activation and increase of Foxp3 expression in T cells from MS (Multiple Sclerosis) patients. We are striving to develop a novel agent to make successful induction of regulatory T cells and control inflammation in autoimmune diseases like multiple sclerosis, etc.
< Bystander T cell functions in autoimmunity, infection, and cancer >

T cells are the central mediators of both humoral and cellular adaptive immune responses. Highly specific receptor-mediated clonal selection and expansion of T cells assure antigen-specific immunity. In addition, encounters with cognate antigens generate immunological memory, the capacity for long-term, antigen-specific immunity against previously encountered pathogens. However, T-cell receptor (TCR)-independent activation, termed "bystander activation", has also been found. Bystander-activated T cells can respond rapidly and secrete effector cytokines even in the absence of antigen stimulation. Recent studies have rehighlighted the importance of antigen-independent bystander activation of CD4+ T cells in infection clearance and autoimmune pathogenesis, suggesting the existence of a distinct innate-like immunological function performed by conventional T cells. We interested in the inflammatory mediators that activate bystander CD4+ T cells and the potential physiological roles of these cells during infection, autoimmunity, and cancer.

Despite the importance of antigen-specific T cells, we show that antigen non-related, bystander memory-like CD4+ T cells also significantly contribute to autoimmune pathogenesis. In our recent study, transcriptome analysis of conventional CD44high CD4 T cells demonstrates that interleukin (IL)-1β- and IL-23-prime T cells that express pathogenic TΗ17 signature genes such as RORγt, CCR6, and granulocyte macrophage colony-stimulating factor (GM-CSF). Importantly, when co-transferred with myelin-specific 2D2 TCR-transgenic naive T cells, unrelated OT-II TCR-transgenic memory-like TH17 cells infiltrate the spinal cord and produce IL-17A, interferon (IFN)-γ, and GM-CSF, increasing the susceptibility of the recipients to experimental autoimmune encephalomyelitis in an IL-1 receptor-dependent manner. In humans, IL-1R1high memory CD4+ T cells are major producers of IL-17A and IFN-γ in response to IL-1β and IL-23. Collectively, our findings reveal the innate-like pathogenic function of antigen non-related memory CD4+ T cells, which contributes to the development of CNS autoimmune diseases like multiple sclerosis.
< Sex-differences in autoimmune diseases >
Sex is a biological factor that contributes to physiological and anatomical differences. Immunological sex differences also exist and cause disparate responses to both self and foreign antigens. In general, males have a greater prevalence and severity of bacterial, fungal and parasitic infectious diseases than females. Women are more susceptible to autoimmune diseases compared to men. Several factors including hormones and the X-chromosome have been suggested to affect the higher prevalence rates of autoimmune diseases in females.
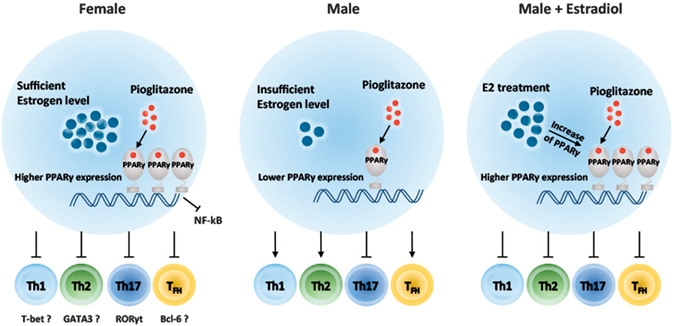
We previously investigated PPARγ expression is higher in T cells from female mice than in cells from males and that pioglitazone effects have a positive correlation with estrogen levels. PPARγ controls TFH responses more sensitively in females with sufficient estrogen levels. This finding suggests that an abnormality in PPARγ activity in T cells might cause a more critical problem for maintaining homeostasis in females than in males. TFH cells are found in B cell follicles and interact with cognate B cells to promote isotype class switching, affinity maturation, and plasma cell differentiation to produce antibodies. Therefore, TFH cells and GC reactions are important drivers of autoimmune disease by supporting autoantibody production. Regulation of TFH cells is now considered to be an important target for treatment of autoimmune diseases35. We discovered PPARγ plays a significant role in the regulation of TFH responses, especially in females, which would be important to prevent autoimmunity and also currently study to find out a novel target to control antibody production. Finally, we would like to understand sex-different immune responses as well as to suggest gender specific therapeutic approaches in autoimmune diseases like lupus, multiple sclerosis, etc.
< Tissue-barrier penetrating peptide and its application to modulate biological events >

Atopic dermatitis (AD) and psoriasis are the most common chronic inflammatory skin diseases. There is a huge unmet medical need to overcome limitations for transcutaneous drug development posed by the skin barrier. We previously aimed to identify a novel transdermal delivery peptide and to develop a transcutaneously applicable immunomodulatory protein for treating AD and psoriasis. We identified astrotactin 1?derived peptide (AP) as a novel transdermal delivery peptide of human origin. It penetrates mouse and human skin tissues with intracellular transduction efficacy in keratinocytes, fibroblasts, and immune cells. We also generated a recombinant therapeutic protein, AP?recombinant protein tyrosine phosphatase (rPTP), consisting of the phosphatase domain of the T-cell protein tyrosine phosphatase conjugated to AP. AP-rPTP inhibited phosphorylated signal transducer and activator oftranscription (STAT) 1, STAT3, and STAT6 in splenocytes and also regulated T-cell activation and proliferation. Finally, we confirmed the in vivo efficacy of AP-rPTP transdermal delivery in oxazolone-induced contact hypersensitivity, ovalbumin-induced AD-like, and imiquimod-induced psoriasis-like skin inflammation models. With a 9-amino-acid novel transdermal delivery peptide, AP, and demonstrated its feasibility for transcutaneous biologic drug development.

Central nervous system (CNS)-infiltrating effector T cells play critical roles in the development and progression of multiple sclerosis (MS). However, current drugs for MS are very limited due to the difficulty of delivering drugs into the CNS. We identified a blood-brain barrier (BBB)-permeable peptide, dNP2, which efficiently delivers proteins into the brain tissue and resident cells through blood vessels by penetrating the tightly organized blood?brain barrier. The dNP2-conjugated cytoplasmic domain of cytotoxic T-lymphocyte antigen 4 (dNP2-ctCTLA-4) negatively regulates activated T cells and shows inhibitory effects on experimental autoimmune encephalomyelitis in both preventive and therapeutic mouse models. Thus, this study demonstrates that dNP2 is a blood?brain barrier-permeable peptide which could be an effective agent for treating CNS diseases such as MS, Alzheimer’s disease, etc.
<Chi3l1 functions in T cells and anti-tumor immunity >
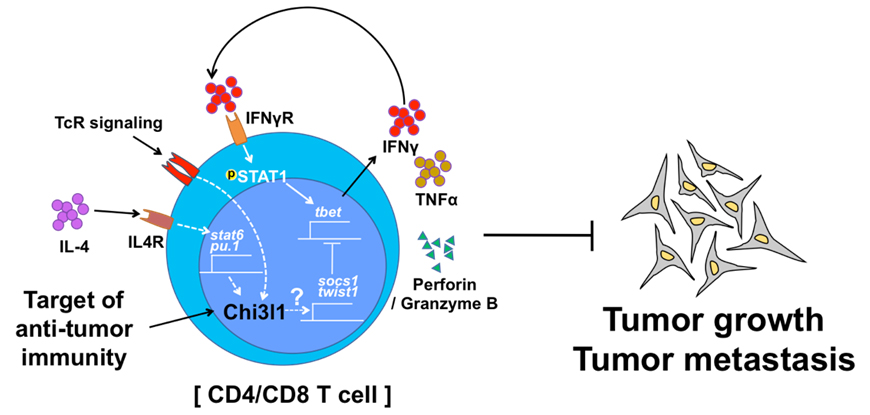
Chitinase-3-like-1 (Chi3l1) is known to play a significant role in the pathogenesis of Type 2 inflammation and cancer. However, the function of Chi3l1 in T cell and its clinical implications are largely unknown. We previously showed that Chi3l1 expression was increased in activated T cells, especially in Th2 cells. In addition, we generated T cell specific Chi3l1 deficient mice. Chi3l1-deficient T cells are hyper-responsive to TcR stimulation and are prone to differentiating into Th1 cells. Chi3l1-deficient Th1 cells show increased expression of anti-tumor immunity genes and decreased Th1 negative regulators. Deletion of Chi3l1 in T cells in mice show reduced melanoma lung metastasis with increased IFNγ and TNFα-producing T cells in the lung. Furthermore, silencing of Chi3l1 expression in the lung using peptide-siRNA complex (dNP2-siChi3l1) efficiently inhibit lung metastasis with enhanced Th1 and CTL responses. Collectively, this study demonstrates Chi3l1 is a novel regulator of Th1 and CTL which could be a therapeutic target to enhance anti-tumor immunity to control melanoma metastasis.







